Page 27 - FoodFocusThailand No.192 March 2022
P. 27
SPECIAL TALK BY FDA
SPECIAL TALK BY FDA
and less than 0.2% of THC by weight . The legalized
1
parts of hemp or cannabis, therefore, can be 4. The qualities and standards of food products containing cannabidiol
processed to make several health products under extract are established as follows:
control of relevant laws and regulations. Regarding (1) Amount of cannabidiol and tetrahydrocannabinol and analytical methods
food industry, using parts of cannabis or hemp in shall comply with the requirements specified in the table.
food produced for commercial purposes is only (2) Use of food additives including extraction solvents shall comply with the
allowed under the general purpose of food and its Notification of MOPH regarding food additives.
qualities and safety are controlled under the Food (3) Pesticide residues shall not exceed the maximum limits as specified in the
Law based on the risk analysis principle. Notification of MOPH regarding food containing pesticide residues.
In order to ensure consumer safety and to (4) Chemical contaminants shall not exceed the maximum limits as specified
establish the appropriate measures and in the Notification of MOPH regarding standards for contaminants in food.
requirements to control quality and standard of food (5) Pathogenic microorganisms shall comply with the Notification of MOPH
products containing cannabidiol extract that could regarding prescribing the quality or standard, principles, conditions and
possibly pose risk to consumers from health- methods of analysis for pathogenic microorganisms in foods.
harming chemical intakes. The Ministry of Public (6) Depending on the case, the quality prescriptions or standards of each specific
Health, by virtue of the Food Act B.E.2522 (1979), food shall be subject to its corresponding notifications of the Ministry of Public
issued the Notification of the Ministry of Public Health.
Health (No.429) B.E.2564 (2021) Re: Food
Products Containing Cannabidiol Extract. The Table: Conditions for the use of cannabidiol extracts in food products
important points are summarized as follows:
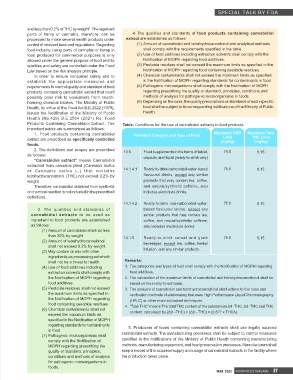
1. Food products containing cannabidiol Permitted Category and Type of Food Maximum CBD Maximum Total
Limit
extract are prescribed as specifically controlled (mg/kg) THC Limit
(mg/kg)
foods.
2. The definitions and scopes are prescribed 13.6 Food supplements in the forms of tablet, 75.0 0.15
as follows: capsule, and liquid (ready-to-drink only)
“Cannabidiol extract” means Cannabidiol
extracted from cannabis plant (Cannabis indica
or Cannabis sativa L.) that contains 14.1.4.1 Ready-to-drink carbonated water-based 75.0 0.15
tetrahydrocannabinol (THC) not exceed 0.2% by flavoured drinks, except any similar
weight products that may contain tea, coffee,
Therefore, cannabidiol obtained from synthetis and natural/synthetic caffeine, also
or chemical reaction is not included in the prescribed includes electrolyte drinks.
definitions.
14.1.4.2 Ready-to-drink non-carbonated water- 75.0 0.15
3. The qualities and standards of based flavoured drinks, except any
cannabidiol extracts to be used as similar products that may contain tea,
ingredient in food products are established coffee, and natural/synthetic caffeine,
as follows: also includes electrolyte drinks.
(1) Amount of cannabidiol shall not less
than 30% by weight. 14.1.5 Ready-to-drink cereal and grain 75.0 0.15
(2) Amount of tetrahydrocannabinol beverages, except tea, coffee, herbal
shall not exceed 0.2% by weight.
(3) May contain or mix with other infusion, and any similar products.
ingredients as processing aid which
shall not be a threat to health. Remarks:
(4) Use of food additives including 1. The categories and types of food shall comply with the Notification of MOPH regarding
extraction solvents shall comply with food additives.
the Notification of MOPH regarding 2. The calculation of the maximum limits of cannabidiol and tetrahydrocannabinol shall be
food additives. based on the ready-to-eat basis.
(5) Pesticide residues shall not exceed 3. The analysis of cannabidiol and tetrahydrocannabinol shall adhere to the rules and
the maximum limits as specified in verification methods of a laboratory that uses High Performance Liquid Chromatography
the Notification of MOPH regarding (HPLC) or other more advanced techniques.
food containing pesticide residues 4. “Total THC” means The total THC content of the substances Δ9 -THC, Δ8 -THC and THC
(6) Chemical contaminants shall not
exceed the maximum limits as content, calculated by (Δ9 –THC) + (Δ8 –THC) + (0.877 x THCA)
specified in the Notification of MOPH
regarding standards for contaminants 5. Producers of foods containing cannabidiol extracts shall use legally sourced
in food
(7) Pathogenic microorganisms shall cannabidiol extracts. The manufacturing processes shall be subject to control measures
comply with the Notification of specified in the notifications of the Ministry of Public Health concerning manufacturing
MOPH regarding prescribing the methods, manufacturing equipment, and food preservation processes. Manufacturers shall
quality or standard, principles, keep a record of the acquired supply and usage of cannabidiol extracts in the facility where
conditions and methods of analysis the production takes place.
for pathogenic microorganisms in
foods.
MAR 2022 FOOD FOCUS THAILAND 27
23/2/2565 BE 12:14
24-28_Special Talk_FDA.indd 27 23/2/2565 BE 12:14
24-28_Special Talk_FDA.indd 27