33
OCT 2017 FOOD FOCUSTHAILAND
SPECIAL TALK
BY FDA
2.3TheAnnouncement of theFood andDrugAdministration on
FoodAdditives (No.3) dated23
rd
JuneB.E.2552 (2009);
2.4TheAnnouncement of theFood andDrugAdministration on
FoodAdditives (No.4) dated5
th
JulyB.E.2553 (2010).
3
Theuseof foodadditivesshallbecompliedwith theNotification
of theMinistryof PublicHealth (No.381) by following thename
of food additive, category or type of food, functional classes and
maximum permitted use level as specify inAnnex 1, and additional
description inAnnex 2.
Annex1:
Theconditionsof use for373 foodadditives, or15,330
maximum permitted levels listed inalphabetical order (A toZ)
Annex 2:
Includes additional descriptionof what listed inAnnex 1,
divided into2 sections;
Section 1: Description of food categories
Section 2: Description of 426notes
4
To use mixture of two or more food additives in the same
functional classes, thecombinationmustnotexceed the lowest
permitted level of food additive. This is replaced by this statement,
“To usemixture of two or more food additives performing the same
functional classes, the sum of themixture proportions obtained by
dividing the amount of each food additive used by the maximum
permitted level for that food additive must not be more than one”
(defined inClause6of theNotificationof theMinistryofPublicHealth
(No.281) as amended by the Notification of the Ministry of Public
Health (No.381)).
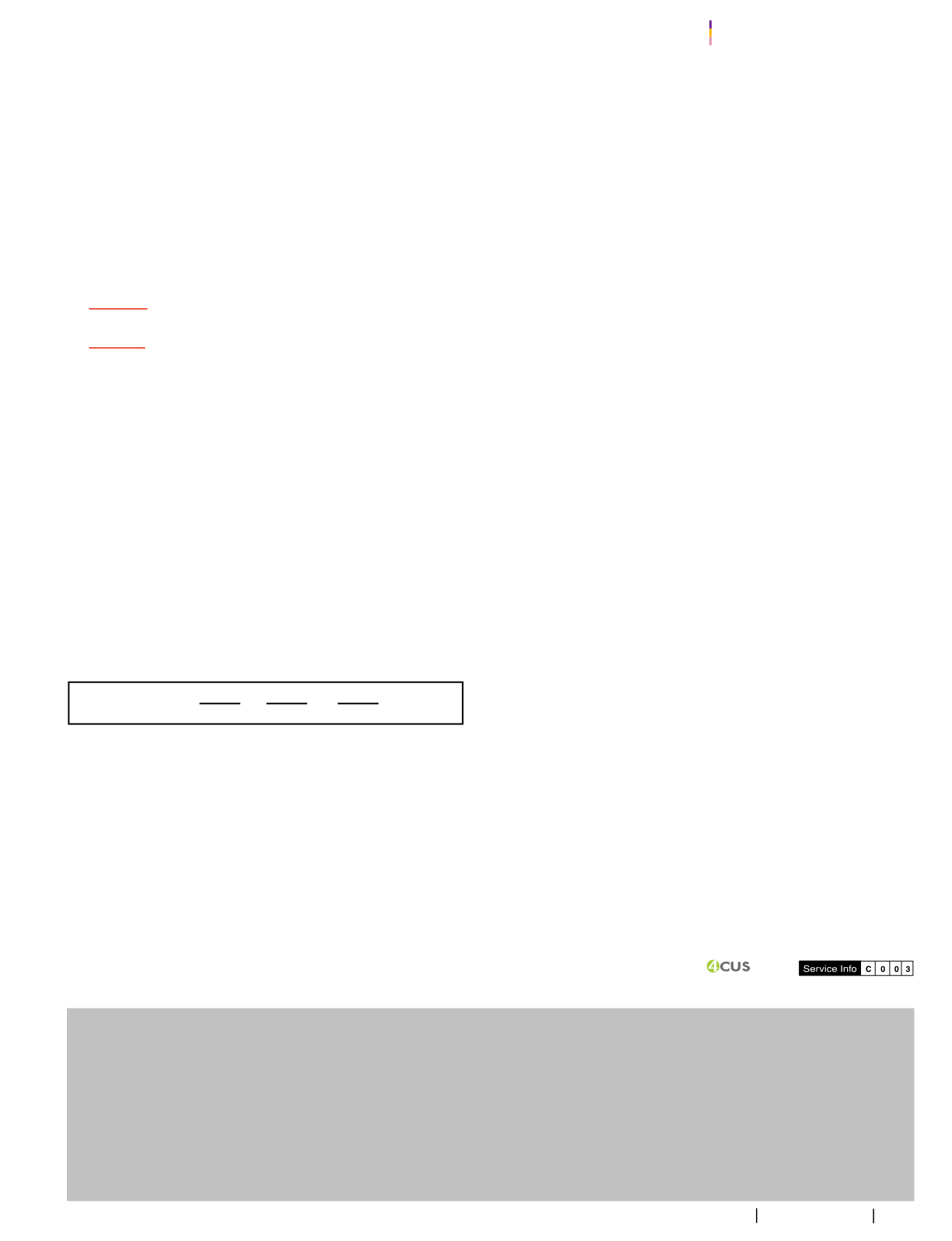
Sum of themixture proportions is calculated inaccordancewith
the followingequation:
MLA=Maximumpermitteduse level of food additiveA (ppm)
MLB=Maximum permitteduse level of foodadditiveB (ppm)
MLC=Maximum permitted use level of foodadditiveC (ppm)
ConcA=Concentration of food additiveA in the food (ppm)
ConcB=Concentration of food additiveB in the food (ppm)
ConcC=Concentrationof food additiveC in the food (ppm)
** Sum of proportions of food additive must not be
more than1**
5
In case the use of food additives is not complied with the
Notification of theMinistry of Public Health (No.381), this use
shall be evaluated for safety aspect and approved by theFood and
DrugAdministration.Thesafetyevaluationshall follow the rules,conditions
and procedures for permission as follows;
• Food additives shall have the qualities or standards according to
the Codex Advisory Specification for the Identity and Purity of Food
Additives (the latest version) or theAnnouncement of theFoodandDrug
Administration;
• Thedietaryexposureof foodadditivesshallbeevaluatedaccording
to the principle approved by theFoodCommittee;
• Technical documents or reliable research publications regarding
theuseof foodadditiveand its function shall be submitted to support the
necessity of using such additives in food;
• Use of food additives shall be complied with the current law and
regulationofat least twocountries,whichhave the reliable riskassessment
system, for example the European Union, Australia, New Zealand, the
UnitedStates ofAmericaand Japan, etc.
6
Theuseof foodadditivesaccording to theNotificationof theMinistry
ofPublicHealth (No.381) shall not enforce to thespecificcontrolled
food or prescribed food quality or standard which have requirements to
useof foodadditivesaddressed in the relevantNotificationof theMinistry
of Public Health, such as Notification of the Ministry of Public Health
(No.83)B.E.2527 (1984),Re:Chocolate,and theNotificationof theMinistry
of Public Health (No.156) B.E.2537 (1994) Re: ModifiedMilk for Infants
andModifiedMilk of follow-upFormula for Infants andChildren.
7
Themanufacturer or food importer who had been obtained a food
additive permission prior to the effective date of theNotification of
theMinistry of PublicHealth (No.381), shall complywith thisNotification
or submit documents and evidence for evaluated safety assessment
according to the rules, conditions and procedures for permission (see in
itemNo.5)within2years from thedateof thisnotificationcome into force.
8
Any person fails to abide by the provision listed in this notification,
it deemed tobeviolated thisnotification; asstipulatedunder section
6(5) of the FoodAct B.E.2522 (1979) shall be fined not more than THB
20,000.Furthermore, refer toarticle25(1)underFoodActB.E.2522 (1979),
foodscontainingveryhighdoseof foodadditive thatmightcauseof illness
in consumer, are classified as impure food products and anyone who
break thesubstantive, shall be imprisonednotmore than2yearsor fined
not more thanTHB 20,000 or both punishment.
9
TheNotificationof theMinistryofPublicHealth (No.381) iseffective
from 21
st
December B.E.2559 (2016) onward, and 21
st
December
B.E.2561 (2018)onward forproducts thatarepreviouslyauthorizedbefore
the enforcement of this notification.
กฎหมายที่
เกี่
ยวข้
อง / Relevant Regulation
ประกาศกระทรวงสาธารณสุ
ข (ฉบั
บที่
379) พ.ศ. 2559 เรื่
อง ยกเลิ
กประกาศกระทรวงสาธารณสุ
ข (ฉบั
บที่
359) พ.ศ. 2556 เรื่
อง ซั
ยคลาเมตลงวั
นที่
3พฤศจิ
กายนพ.ศ. 2559 สื
บค้
นได้
จาก
ประกาศกระทรวงสาธารณสุ
ข (ฉบั
บที่
380) พ.ศ. 2559 เรื่
อง ยกเลิ
กประกาศกระทรวงสาธารณสุ
ข (ฉบั
บที่
360) พ.ศ. 2556 เรื่
อง สตี
วิ
ออลไกลโคไซด์
ลงวั
นที่
3พฤศจิ
กายนพ.ศ. 2559 สื
บค้
นได้
จาก
ประกาศกระทรวงสาธารณสุ
ข(ฉบั
บที่
381)พ.ศ.2559 เรื่
องวั
ตถุ
เจื
อปนอาหาร(ฉบั
บที่
4)ลงวั
นที่
3พฤศจิ
กายนพ.ศ.2559สื
บค้
นได้
จากhttp://www.ratchakitcha.soc.go.th/DATA/PDF/2559/E/298/3.PDF
1
+
+
+...
>_
ConcA ConcB ConcC
MLA MLB
MLC
Notificationof Ministry of PublicHealth (No.379) B.E. 2559 (2016) Re: To repeal NotificationofMinistry of PublicHealth (No. 359) B.E2556 (2013) Re: Cyclamates.
dated3
rd
November B.E. 2559 (2016). Available from:
NotificationofMinistry of PublicHealth (No. 380) B.E2559 (2016) Re: To repeal Notificationof the Ministry of PublicHealth (No. 360) B.E2556 (2013) Re: Steviol glycosides.
dated3
rd
November B.E. 2559 (2016). Available from:http://www.ratchakitcha.soc.go.th/DATA/PDF/2559/E/298/2.PDF
Notificationof Ministry of PublicHealth (No.381) B.E. 2559 (2016) Re: FoodAdditives (No.4). dated3
rd
November B.E. 2559 (2016).
Available from:http://www.ratchakitcha.soc.go.th/DATA/PDF/2559/E/298/3.PDF


